Metalic rainbows all around us

Have you ever looked closely at metal cutlery after sticking it into hot food? An oil slick on the road? The strange sheen on a beetle?
These colours, sometimes called oil slick or neochrome, have a common origin. I wondered where the colour came from and found a couple cool things you may not have noticed about these colours along the way.
If you want to explain a colour, light must be the place to start.
It’s easy to take light for granted and forget just how incredible it is. For one thing, the microwaves that cook your food, the light coming from your screen as you read this and the gamma radiation in a nuclear disaster are all the same. They are light of different wavelengths. This is also how we get colours. Our eyes can detect light with a wavelength between about 380 and 700 nanometres. 700 nm is red and ~400 nm is blue. But if light is a wave*, what happens when two light waves meet?
If you’ve ever sat on a harbour and watched the waves reflect off the concrete you have seen interference patterns before. Sometimes two troughs meet and you get a deeper trough, other times a trough and peak meet and cancel. The same thing can happen with light. Consider a heated metal:
A light wave is coming towards a heated (now neochrome) metal. First it hits the metal oxide layer on top. Burning is the reaction of Oxygen with some substance and heating the metal has left a layer of metal oxide on the surface. Some of the light is reflected from this thin layer but the rest makes it through and reflects off the pure metal. The light that travelled through the thin-film was slowed down and so when it meets the light being reflected off the surface again, they are out of phase (the peaks and troughs no longer line up perfectly). Some frequencies undergo destructive interference and become dimmer and others constructive and are brighter.

Whether a particular wave is cancelled or enhanced depends on the thickness of the layer, the frequency of the light and the angle the light was incident at. These three variables explain some of interesting effects we can observe:
Cool thing 1: Colour varies all over the surface of a heated metal.
When you see those complicated patterns of colour on a heated spoon, that’s due to variation in the thickness of that oxide layer. This is demonstrated in this picture of a suspended soap film. Under gravity the film thickens as you go down the image. You can see how this changes the colour observed.
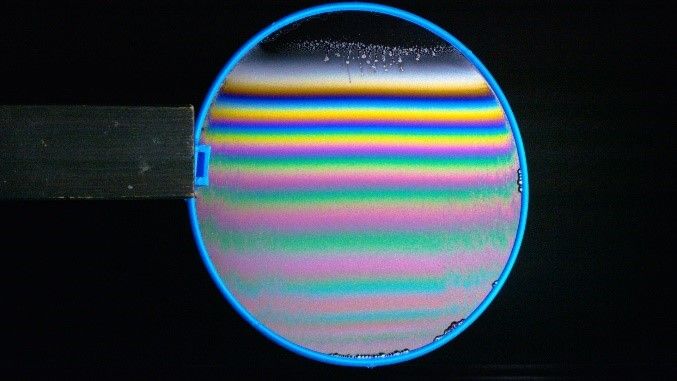
Cool thing 2: As you shift the object or your perspective the colour shifts and changes as well.
The fact the colour is dependent on what angle the light strikes it is why the rainbow shifts as you change perspective. You could see this as being similar to increasing the thickness of the layer since the light has to travel further through the thin-film if the angle of incidence is shallower.
Cool thing 3: You see metallic colours as opposed to the normal rainbow.
The reason you see golds, magentas and metallics rather than the usual rainbow is because only particular narrow bandwidths get enhanced while others are diminished due to the dependence on frequency, so you don’t have a single enhanced frequency but many, these combine to form the metallic appearance.
The thin-film effect is surprisingly ubiquitous. It even comes up in minerals where it is called the schiller effect. The next time you see a metallic rainbow you might want to guess what could be acting as the film. For example, this neochrome knife?

These are made by applying an incredibly thin coat of something like titanium nitride.
Happy colour hunting.
*In fact, light behaves like both a wave and a particle.
Credit for images:
Flikr, Andy Rogers and Caramosca,
Wikipedia, Thin film interference
Harvard: https://sciencedemonstrations.fas.harvard.edu/presentations/thin-film-interference



Comments ()